What Are the Common Units for Expressing Solution Concentration
There are multiple units of concentration. The concentration of a solution is a macroscopic property represents the amount of solute dissolved in a unit amount of solvent or of solution and can be expressed in a variety of ways qualitatively and quantitatively.
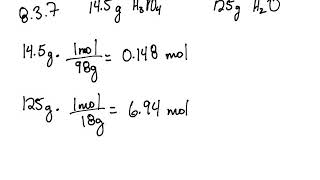
8 3 Concentrations Of Solutions Problems Chemistry Libretexts
As we have seen there are different ways of expressing the concentration of a solutions.

. Most commonly the concentration of a solution is expressed as mass or volume percentage and molarity or molality although molarity is temperature dependent it is used quite commonly used to express the concentration of a solution. Common Units Used to Express Solution Concentration Molarity M Molality m mole fraction x mole percent mol percent by mass or volume parts per million ppm by mass or volume and parts per billion ppb by mass or volume Presence of a Nonvolatile Solute in a Liquid Results in. The two most common ways of expressing concentration are molarity and molality.
It is the number of moles of solute dissolved in one litre of a solution. Which unit you use depends on how you intend to use the chemical solution. Molarity M moles soluteliter of solution Normality N equivalents of soluteliter of solution Weight Wt mass of solutemass of solution x 100 Parts per million ppm mass of solutemass of solution x 106.
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. Parts by mass h. We have step-by-step solutions for your textbooks written by Bartleby experts.
Molarity of the solution is the number of moles of the solute present in 1 liter of the solution. Parts by volume g. Ways of expressing concentrations of solutions in chemical units.
The unit of molarity is mol kg -1. Molarity M Parts per million ppm. One of the most commonly used methods for expressing the concentrations is molarity.
Find step-by-step Chemistry solutions and your answer to the following textbook question. Molarity The molar concentration M of a solution is defined as the number of moles of solute n per liter of solution ie the volume V solution. Similarly other terms such as ppm TDS of water molar and normal laboratory reagents are used to express the concentration of the solution.
There are a number of ways to express the relative amounts of solute and solvent in a solution. Chemistry questions and answers. It is used to express the concentration of the solution.
The unit to express molarity is moles per liter which is also called as molar. The common units to express the concentration of solution. Molality is the most convenient method to express the concentration of solutions because it involves the mass of liquids rather than their volumes.
The formality of a solution is the number of gram-formula weights of the solute contained in one liter of the solution. Molality m Mass of solute Mass of solvent in Kg Normality N. Common units for expressing solution concentration are- Mole percent- It is defined as the total moles of substance in a compound multiplied by 100It is used to measure concentration of solute and solvent in solution.
Concentration of solution Solute mass in gram Solution volume in liters If the unit of volume is in mL then the overall formula should be multiplied by 1000. And so our units for concentration are typically similarity. There are a number of different ways of expressing solute concentration that are commonly used.
Molarity of any solution is number of moles of solute per liter of solution. The formula for molality is given as follows. So our overall rate in our changing quantity over time is going to be big M over seconds.
The concentration of a solution gives the amount of solute present in a given quantity of solvent. Check all that apply. Molarity Number of moles of solute Volume of solution in liter.
A solution can be qualitatively described as. 4 Ways to Express Concentrations in Solutions If you were drinking Kool-Aid you might describe the solution as dilute or concentrated as a means to express concentration. This page describes calculations for four different units used to express concentrationPercent.
Thus if one gram molecule of a solute is present in 1 kg of the solvent the concentration of solutions is said to be one molal. What are the common units for expressing solution concentration. The most common units are molarity molality normality mass percent volume percent and mole fraction.
Molarity can be used to calculate the volume of solvent or the amount of solute. It is the number of moles of solute contained in 1000 ml of solution. Textbook solution for Chemistry.
So this is going to be our unit. M n Vsolution M n V solution The units of molarity are molL often abbreviated as M. Molality m The molality is expressed as the number of moles of a solute contained in 1000 gm of a solvent.
Concentration amount solute part amount solvent whole Chemists can express concentrations in various ways including. Qualitative Expressions of Concentration. So were talking about the change in the concentration of something over time.
It is a commonly used method for expressing concentrations. Parts by volume- It is defined View the full answer. What are the common units for expressing solution concentration.
Some of these are listed below. Calculate the molar concentration of 2000 ppm of Pb 2 A. A Molecular Approach 4th Edition 4th Edition Nivaldo J.
Concentration of solution Solute mass in gram Solution volume in mL X 1000 The strength of the solution can be defined as the concentration of solution in grams per liter. The molarity of a solution is the number of moles of the solute contained in one liter of the solution. So moles per leader and our units for time were going to be using seconds.
Suppose a solution of ethanol is marked 025 M this means that in one litre of the given solution 025 moles of ethanol is dissolved. Tro Chapter 13 Problem 16E. PPM M x M.
Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution.

11 1 Composition Of Solutions Chemistry Libretexts
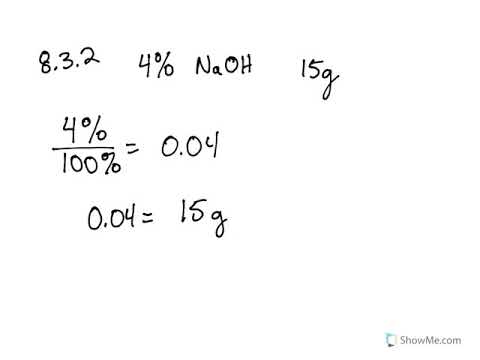
8 3 Concentrations Of Solutions Problems Chemistry Libretexts

8 3 Concentrations Of Solutions Problems Chemistry Libretexts
11 1 Composition Of Solutions Chemistry Libretexts

Measuring Concentration Ck 12 Foundation

Hsp70 Chaperone Blocks A Synuclein Oligomer Formation Via A Novel Engagement Mechanism Journal Of Biological Chemistry

8 3 Concentrations Of Solutions Problems Chemistry Libretexts
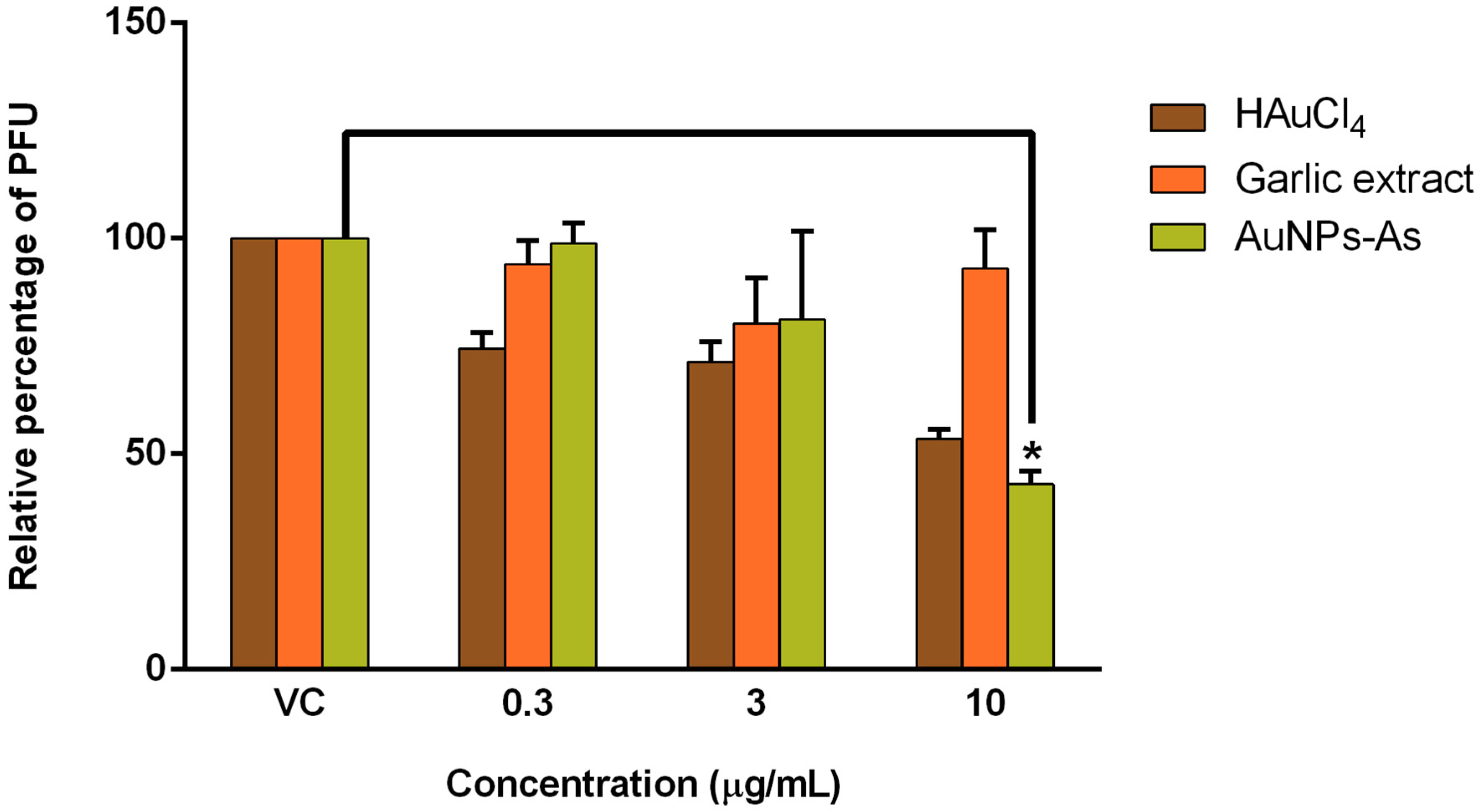
Viruses Free Full Text Virucidal Activity Of Gold Nanoparticles Synthesized By Green Chemistry Using Garlic Extract Html

3 4 Other Units For Solution Concentrations Chemistry
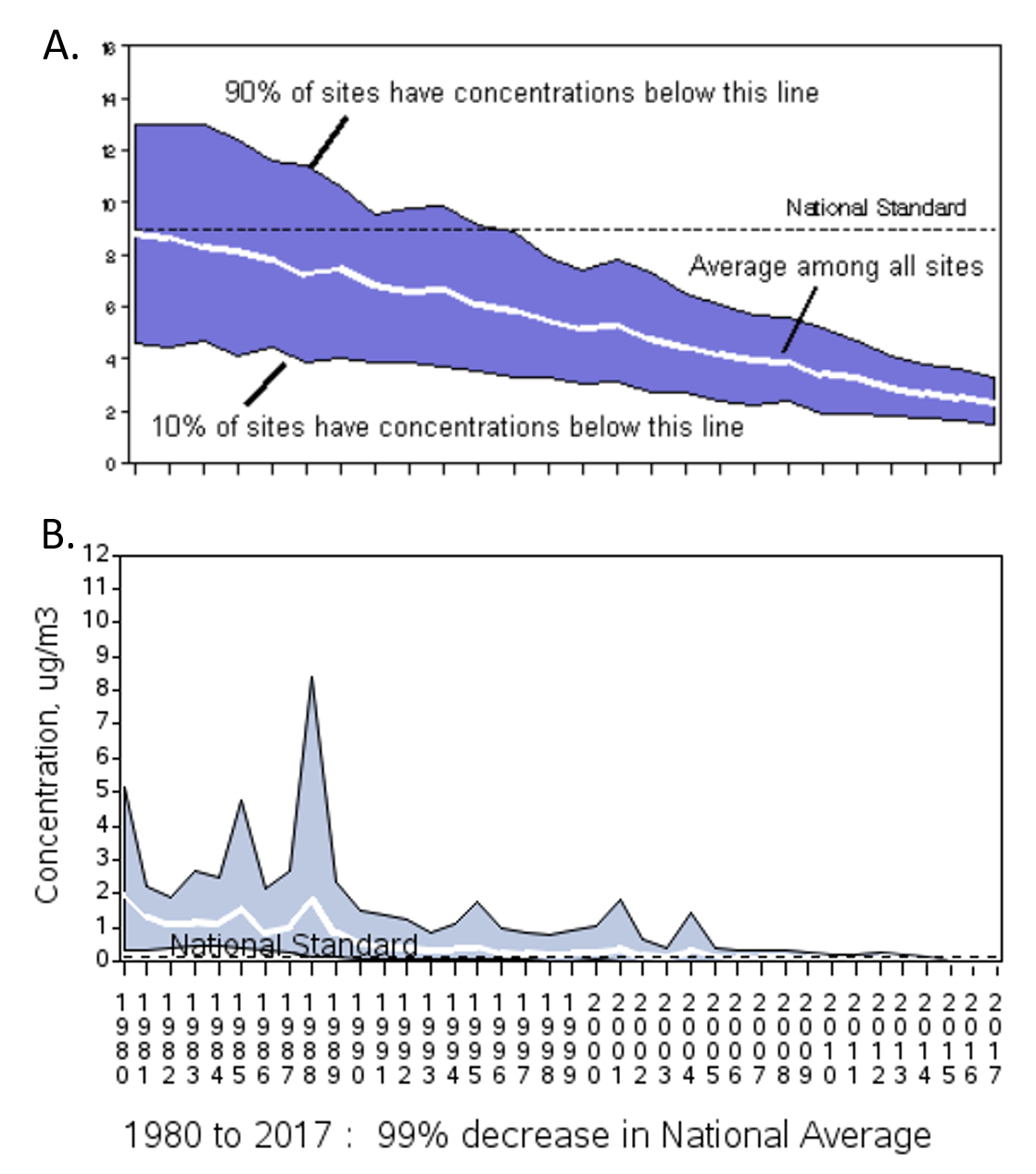
Ch104 Chapter 7 Solutions Chemistry

3 4 Other Units For Solution Concentrations Chemistry
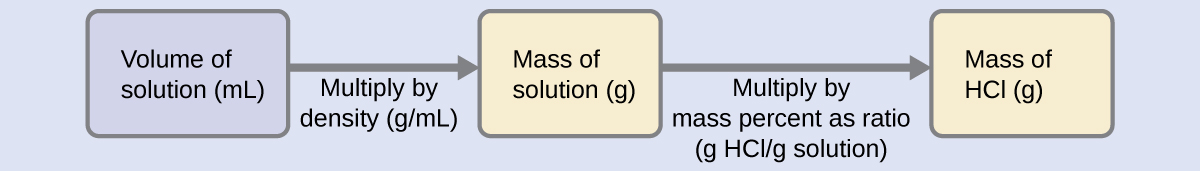
3 4 Other Units For Solution Concentrations Chemistry

Ch104 Chapter 7 Solutions Chemistry

8 3 Concentrations Of Solutions Problems Chemistry Libretexts
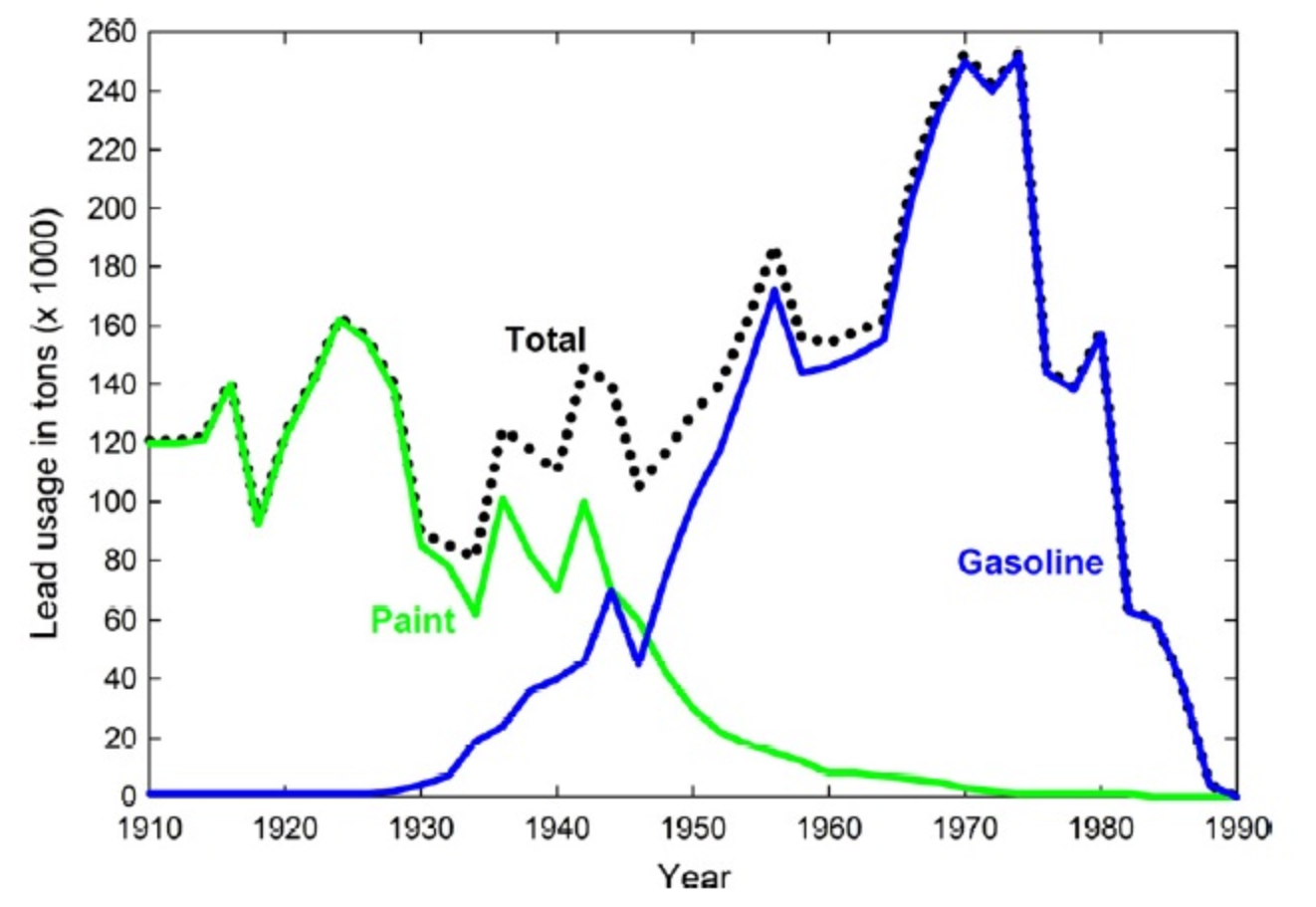
Ch104 Chapter 7 Solutions Chemistry
3 4 Other Units For Solution Concentrations Chemistry

Standard Curve An Overview Sciencedirect Topics

8 3 Concentrations Of Solutions Problems Chemistry Libretexts
Comments
Post a Comment